Preparation and Characterization of Carboxylterminated for Fusion-bonded-epoxy Powder Coating
Related Product for Sale: FBE Powder Coating
Related Articles: FBE Powder Coating
Preparation and Characterization of Carboxylterminated Poly (butadiene-co-acrylonitrile) -epoxy Resin Prepolymers for Fusion-bonded-epoxy Powder Coating
1 Introduction
Fusion-bonded-epoxy (FBE) powder coatings which were first developed by 3M Co., are used widely when long-term corrosion protection is critical such as in the oil, metal, gas and water pipelines industries. However, the performance requirements for FBE powder coatings are challenging because of their high cross-linking density. The inherent brittleness of cured coatings is one of the major obstacles preventing wider application for epoxies in industries. Therefore, it may be possible to improve the performance of FBE coatings by increasing the toughness of the coating.Many toughening methods have been used for toughening epoxy systems, often in composite applications, including rubber, elastomer, thermoplastic, copolymer, nanoparticle modified epoxies and combinations of the above.
Although there was many researches into toughening modifications of epoxy systems, the majority of the
studies involved the chemical modification of epoxy resin with reactive liquid rubber, particularly
carboxyl-terminated butadiene-co-acrylonitrile (CTBN). McGarry et al used CTBN of molecular weight 3000 and various DGEBA epoxies cured with piperidine. Kinloch et al revealed a dynamic dependency in the DGEBA/CTBN/piperidine system by calculating the impact fracture toughness at different strike velocities and obtaining a near two-fold increase in toughness. CTBN could be introduced to the epoxy
systems such as diglycidyl ether of bisphenol-A (DGEBA) epoxy resins. When such epoxy resins are
cured together with the liquid rubber, the toughness of the domains can be improved by absorbing the impact energy. It is well known that the cured resins include two phase systems[26] in which the liquid rubber is dispersed in a matrix of epoxy with a spherical domain structure or a continuous structure.
Thus far, the toughening of epoxy resins has mainly focused on the liquid epoxy resins, and little research has focused on toughening solid epoxy resins.In this paper, we prepared CTBN-EP prepolymers
without using any organic solvents. Then FBE powder coatings composites fi lled with CTBN-EP prepolymers were produced. Based on the mechanical properties and morphological analysis, attempts were made to analyze toughening mechanisms prevailing in the phase separated matrix. Analysis of the structure property relationship of the CTBN-EP system is a new endeavor to the best of our knowledge. Thus, this novel toughening technology can expand the application areas of FBE powder coatings in industry.
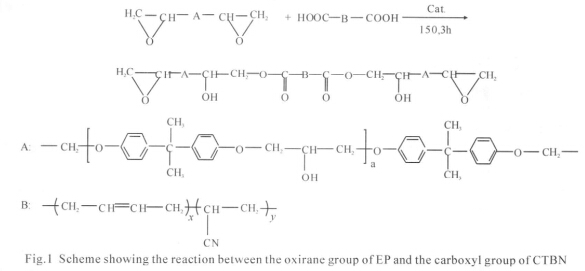
2 Experimental
2.1 Materials
The epoxy resin used was solid diglycidyl ether of bisphenol A (DGEBA) (DOW, DER663) with an epoxide equivalent weight of 750-900. Liquid, carboxyl-terminated poly(butadiene-co-acrylonitrile) (CTBN) (Emerald, Hypro 1 300×1323) with an acrylonitrile content of 26% was used. Triphenyl phosphine was used as a catalyst in this system. The curing agent (HTP-305) was a phenolic. Phenolic epoxy resin (GT7255) was purchased from HUNTSMAN Co.,Pigment(L6900), which was supplied by BASF Co., Degassing agent and leveling agent were purchased from Aisitelun.
2.2 Synthesis and characterization of CTBNEP prepolymers
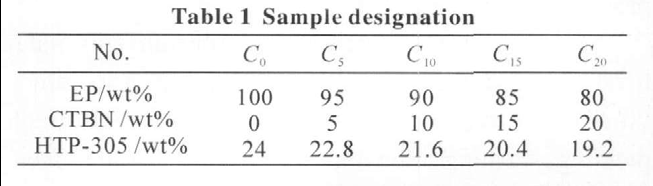
Stoichiometric amounts of epoxy resins, CTBN and catalyst were put in a flask which as heated and stirred mechanically stirring at 150 ℃ for 3.0 h. The reaction was stopped when the acid value dropped to 0. The prepolymers were marked as C0, C5, C10, C15, and C20 (subscripts are the contents of the CTBN). The possible reaction is shown in Fig.1.
FTIR spectroscopy was used to characterize the structures. FTIR spectra were recorded by a FTLA2000-104 spectrophotometer in the wavelength range of 4 500–500 cm−1 (ABB Bomem of Canada). The molecular weights and molecular weight distribution of CTBN-EP prepolymers were determined by GPC. Tetrahydrofuran (THF) was used as an eluent at a fl ow rate of 1.0 mL/min. The column system was calibrated using monodispersed standard polystyrenes.
2.3 Preparation and characterization of curing films
Five curing fi lms containing 0wt%-20wt% CTBN were prepared. The calculated quantities of DGEBA (as per formulation given in Table 1) and HTP-305 were stirred at 120 ℃ for 10 min to get a homogeneous mixture. The mixture were poured into a preheated iron mold cured in a hot air oven at 180 ℃ for 10 min and then post cured for 30 min at 200 ℃.
The tensile tests were performed on a KD111-5 machine (KaiQiang Co., Ltd., China) at a cross-head speed of 1 mm/min. The values were taken from an average of three samples according to GB/ 2568-81. The elongation at the breaking point of the specimen was evaluated. The impact strength of the specimen was determined on a MZ-2056 machine using rectangular specimens of 40 mm × 10 mm × 2 mm. The tests were carried out at room temperature and values were taken from an average of three samples according to GB/ T2571-1995.
The glass transition temperatures of the curing films were determined using a dynamic mechanical analyzer (DMA) .The measurements were carried out at a heating rate of 2 ℃/min from -90 ℃ to 180 ℃ at a fi xed frequency level of 1 Hz. The storage modulus, loss modulus and loss factor were obtained using dual cantilever mode with a sample of size 30 mm × 10 mm × 2 mm.
Scanning electron microscopy (SEM) was performed (Quanta-2000 model SEM, FEI of Dutch) with an electron voltage of 10 kV. The samples were
fractured under liquid nitrogen and first treated with toluene to extract the rubber phase before being dried under vacuum. The size and distribution of dispersed particles were determined by means of taking a semiautomatic image.
The percent weight loss and thermal degradation characteristics of prepared samples were evaluated by a thermogravimetric analyzer (TGA) recorded on Instrument (METTER Toledo of Switzerland). The amount of sample taken was approximately 5-10 mg in a platinum sample pan. The heating rate in each run was kept at 10 ℃/min and the temperature range was ambient to 800 ℃.

 D5 Creation
D5 Creation
Comments are Closed